The initial evaluation should be focused on screening for specific risk factors that would alter the appropriate treatment . The predictors that have been identified as being associated with an increased risk of complications during follow-up are tamponade, recurrences and constriction. Other predictors, called minor risk factors, should also be considered.
These are based on expert opinion and literature review, including acute pericarditis associated with immunodeficiency, trauma, anticoagulant therapy and myocarditis . Any clinical presentation that may suggest an underlying aetiology (e.g. a systemic inflammatory disease) or with at least one predictor of poor prognosis warrants hospital admission and an aetiology search. Patients without signs and symptoms of systemic inflammatory disease can be managed as outpatients with empiric anti-inflammatories and short-term follow-up after one week to assess the response to treatment. In patients identified with a cause other than viral infection, specific therapy appropriate to the underlying disorder is indicated, and the epidemiological background (high vs. low prevalence of TB) should be considered . The two most serious complications of pericarditis are an effusion causing cardiac tamponade and constrictive pericarditis.
Cardiac tamponade occurs when a pericardial effusion becomes large enough to impair contraction of the heart. Symptoms of cardiac tamponade include dyspnea, chest discomfort, fatigue, fluid accumulation in the body , and low blood pressure . In severe cases, cardiac tamponade can impair cardiac function to the point where it compromises delivery of blood and oxygen to the organs .
Constrictive pericarditis is a consequence of chronic pericardial inflammation and is characterized by scarring and loss of elasticity of the pericardium. The main symptoms of constrictive pericarditis are dyspnea, edema, shortness of breath when lying flat and chest pain. Overall, most patients can live a productive life with a very low risk of mortality related to recurrent pericarditis. The pericardium is a double-walled, fibrous sac typically containing 20–50 mL of fluid surrounding the heart and great vessels. In addition to its known anatomical and physiological functions, the pericardium also serves an active immunological role that is the focus of interest pertaining to inflammatory cardiac conditions such as pericarditis and myocarditis.
This is a cross-sectional study of 29 published cases of acute myopericarditis following COVID-19 mRNA vaccination. The most common presentation was chest pain within 1–5 days after the second dose of mRNA COVID-19 vaccination. Cardiac magnetic resonance imaging revealed late gadolinium enhancement consistent with myocarditis in 69% of cases. Most patients were treated with non-steroidal anti-inflammatory drugs for symptomatic relief, and 4 received intravenous immune globulin and corticosteroids. We speculate a possible causal relationship between vaccine administration and myocarditis. The data from our analysis confirms that all myocarditis and pericarditis cases are mild and resolve within a few days to few weeks.
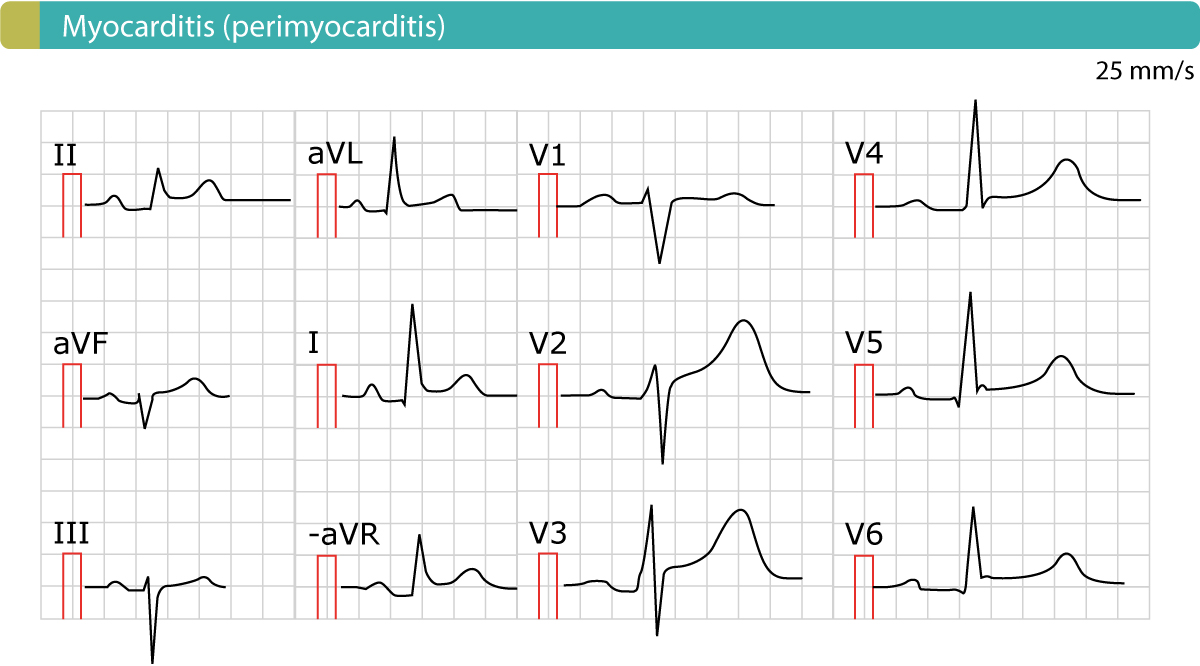
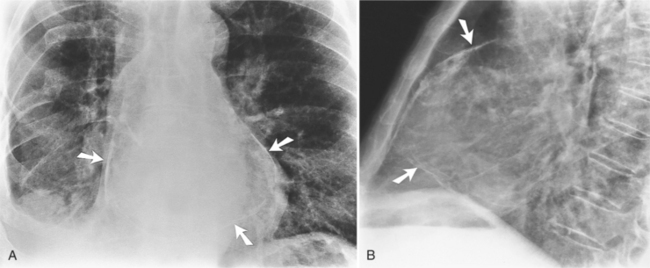
Chest X-ray is frequently normal unless there is a sizeable pericardial effusion. Inflammatory markers (erythrocyte sedimentation rate and C-reactive protein) are often raised and there may also be slight elevations of troponin if there is associated myopericarditis. More significant elevations and/or clinical or echo features of left ventricular dysfunction should prompt a consideration of myocarditis instead or so-called perimyocarditis where myocardial involvement predominates. If there is a period of intervening remission lasting more than 4–6 weeks, the term recurrent is used.
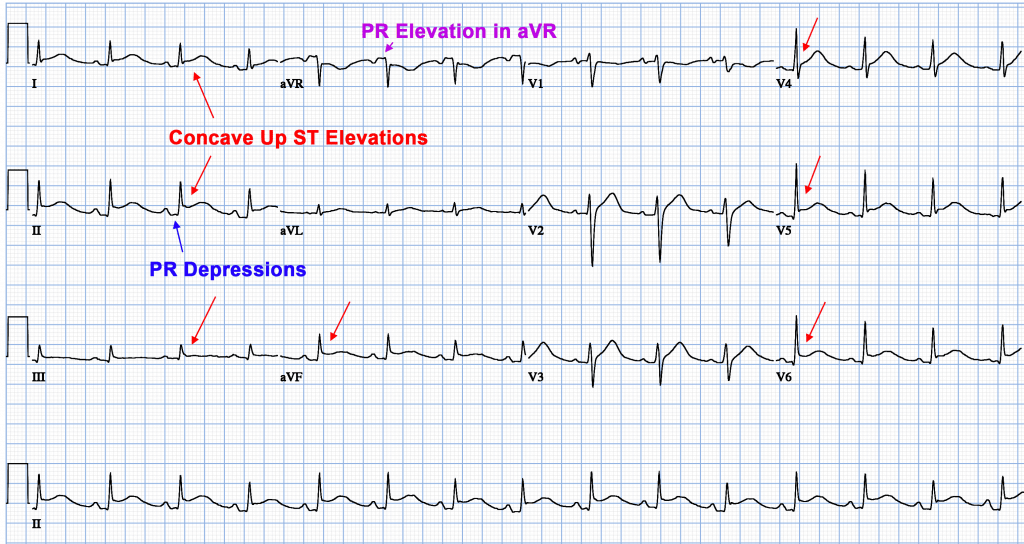
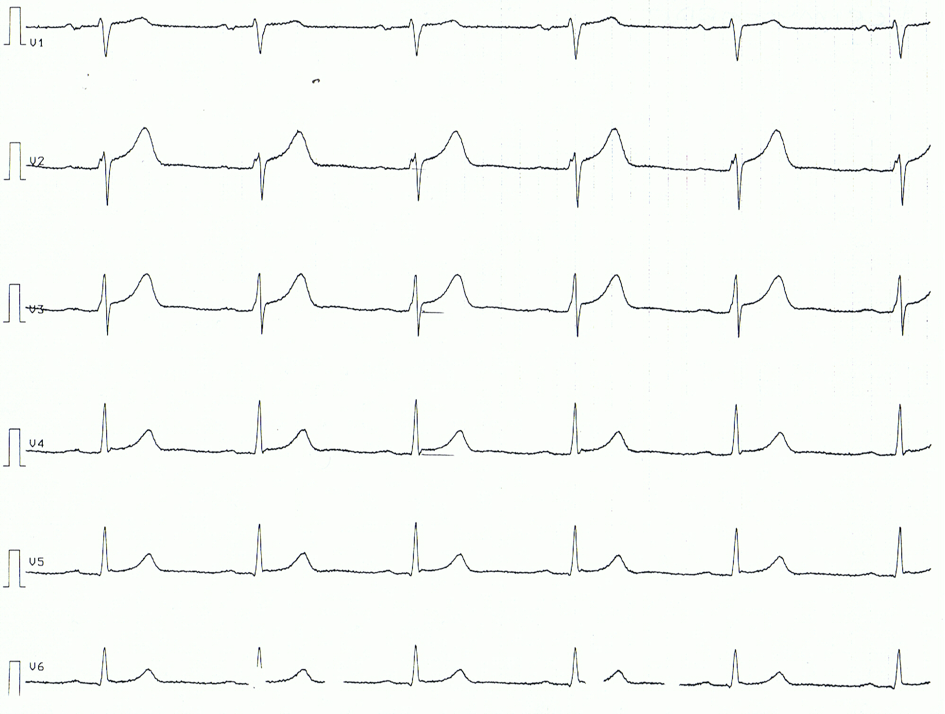
These terms are relevant to therapeutic decision making and investigation pathways. After gathering a complete history and performing an appropriate physical examination, certain tests will be performed in all patients with suspected pericarditis. An electrocardiogram, which measures electrical activity of the heart, might show characteristic changes associated with pericarditis and can help rule out other cardiac causes of chest pain.
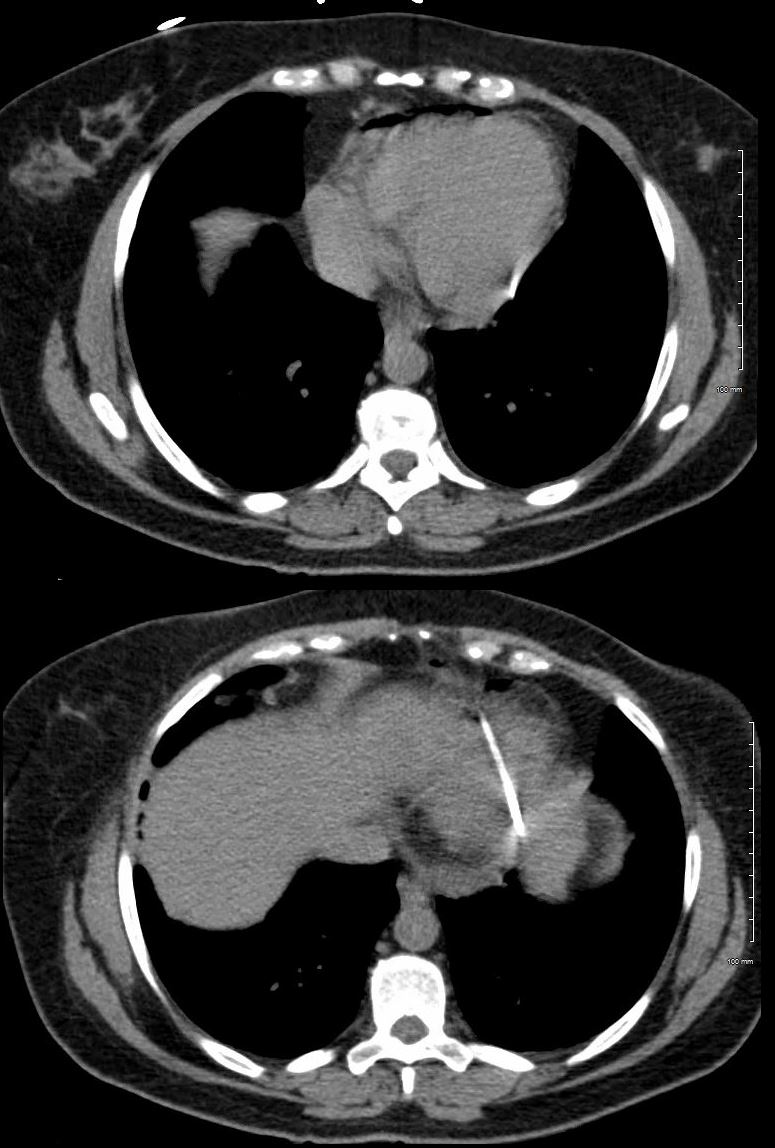
Blood levels of troponin, a protein that is released into the blood following damage to cardiac muscle, are also useful to differentiate pericarditis from other heart conditions. Troponin levels are usually normal in isolated pericarditis but are elevated in myopericarditis, myocarditis and myocardial infarction. Other routine laboratory tests include a complete blood count, which can show an increase in white blood cells due to inflammation, and certain inflammatory markers, namely, C-reactive protein and erythrocyte sedimentation rate . Routine imaging tests include a chest X-ray, which is usually normal in pericarditis but can show signs of an alternative diagnosis or a pericardial effusion if it is large. An echocardiogram, an imaging method that uses sound waves to evaluate the anatomy and function of the heart and pericardium, is also routinely performed in patients with suspected pericarditis.
Myocarditis and pericarditis are inflammatory conditions of the heart commonly caused by viral and autoimmune etiologies, although many cases are idiopathic. Emergency clinicians must maintain a high index of suspicion for these conditions, given the rarity and often nonspecific presentation in the pediatric population. Children with myocarditis may present with a variety of symptoms, ranging from mild flu-like symptoms to overt heart failure and shock, whereas children with pericarditis typically present with chest pain and fever. The cornerstone of therapy for myocarditis includes aggressive supportive management of heart failure, as well as administration of inotropes and antidysrhythmic medications, as indicated. The acute management of pericarditis includes recognition of tamponade and, if identified, the performance of pericardiocentesis. Medical therapies may include nonsteroidal anti-inflammatory drugs and colchicine, with steroids reserved for specific populations.
This review focuses on the evaluation and treatment of children with myocarditis and/or pericarditis, with an emphasis on currently available medical evidence. Myocarditis is an uncommon cause for dilated cardiomyopathy that disproportionately affects children and young adults, leading to a substantial burden of disability and premature cardiovascular death. Until the last decade, the incidence and prevalence of myocarditis could only be estimated from case series drawn mostly from tertiary care medical centres. In contrast, pericarditis is a relatively common cause of chest pain that usually responds to anti-inflammatory treatment. However, pericarditis can lead to a disabling syndrome of chronic or recurrent chest pain, heart failure from pericardial constriction, and, rarely, death. This aim of this chapter is to summarize the current state of knowledge on the global burden of myocarditis and pericarditis.
Characteristic clinical findings in pericarditis include pleuritic chest pain and pericardial friction rub on auscultation of the left lower sternal border. Electrocardiography may reveal diffuse PR-segment depressions and diffuse ST-segment elevations with upward concavity. The most common aetiologies of pericarditis are idiopathic and viral, and the most common treatment for these are nonsteroidal anti-inflammatory drugs and colchicine.
The complications of pericarditis include pericardial effusion, tamponade and myopericarditis. The presence of effusion, constriction or tamponade can be confirmed on echocardiography. Tamponade is potentially life-threatening and is diagnosed by the clinical findings of decreased blood pressure, elevated jugular venous pressure, muffled heart sounds on auscultation and pulsus paradoxus. Acute pericarditis presents in a variety of ways, depending upon the underlying etiology. Pericarditis with an infectious etiology may be preceded by signs and symptoms of infection; by flulike symptoms, for a viral etiology; or, for an autoimmune or malignancy etiology, by exacerbation of symptoms of the underlying disorder. Such patients should be closely monitored for signs of cardiac tamponade, a potentially lethal complication of pericarditis.
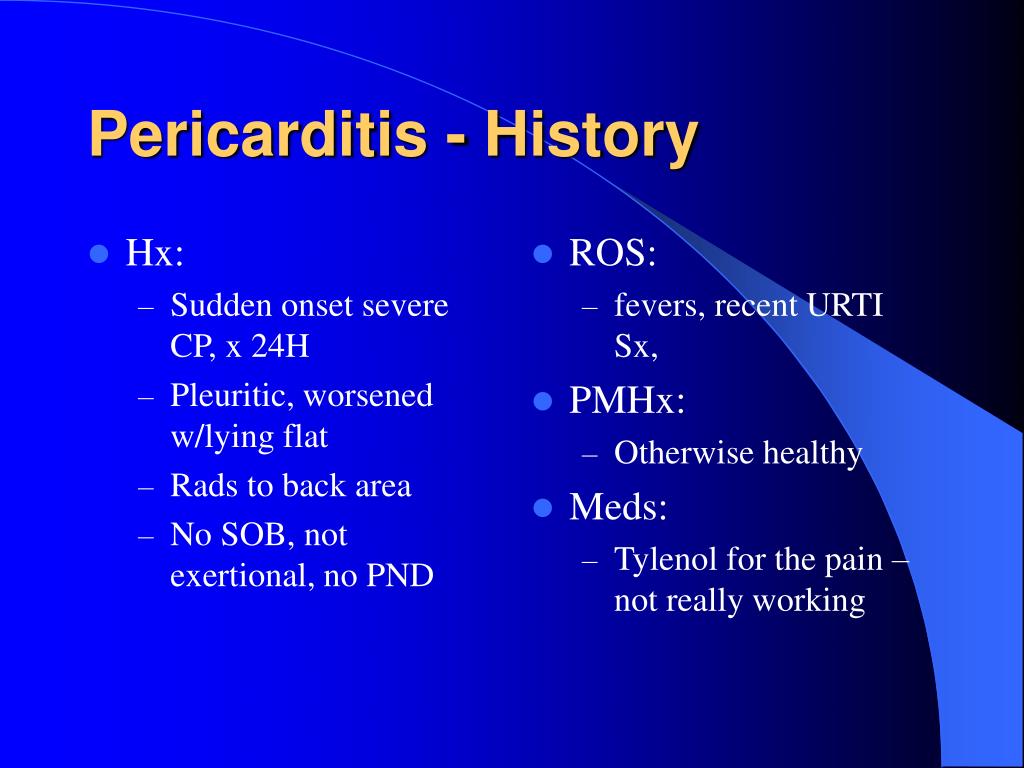
An essential part of the physical examination is auscultation of the heart using a stethoscope; in some patients, a characteristic scratching sound, known as a pericardial friction rub, may be heard. The physical exam can also show signs of cardiac tamponade or constrictive pericarditis, such as dyspnea, distended neck veins, edema, or low blood pressure. The main symptom of pericarditis is chest pain, which is present in the vast majority of affected individuals. Typically, the pain is described as sharp and worse when coughing or taking a deep breath .
The typical pain seen in pericarditis is also worse when lying down and is partially relieved when leaning forward. Other symptoms that are associated with pericarditis include shortness of breath , fever, fatigue, malaise and the sensation of an irregular heartbeat . Another feature frequently seen in pericarditis is accumulation of fluid in the space between the heart and the pericardium , which is known as a pericardial effusion.
The symptoms seen with a second or subsequent episode of recurrent pericarditis are often similar to the first event, although they tend to be less severe with recurrences. Although the symptoms of pericarditis can significantly affect quality of life, affected individuals typically have no symptoms between episodes. The number of episodes of recurrent pericarditis varies greatly between patients. Myocarditis is inflammation of the middle layer of the wall of the heart muscle, the myocardium, and it can temporarily or permanently weaken the heart muscle and the heart's electrical system, which keeps the heart pumping regularly. Approximately 10 to 20 per 100,000 people are diagnosed with myocarditis in the U.S. annually, and in children, the incidence is 1 to 2 per 100,000.
Although many cases resolve on their own or with treatment, leading to a full recovery, severe myocarditis can lead to heart failure, abnormal heart rhythms, shock and sudden death. Signs and symptoms of myocarditis include fatigue, shortness of breath, fever, chest pain and palpitations . In every patient with pericarditis, restriction of physical activity is recommended until symptoms have resolved and inflammatory markers have normalized. If a systemic disease is identified as the cause of pericarditis, therapy should be focused on treating the underlying condition.
For instance, antibiotics will be required for a patient with tuberculosis, and chemotherapy or other treatments will be required in a patient with neoplastic pericarditis. Another important consideration is whether the affected individual needs to be admitted to the hospital or can be treated as an outpatient. Although most patients can be treated outside the hospital, patients with high-risk features are usually admitted. These features include fever, a slow onset of disease without sudden onset of chest pain, presence of a large pericardial effusion, use of immunosuppressant medications or blood thinners or high troponin levels .
Various infections (viral, bacterial, fungal, and tick-borne) have been known to cause the inflammation of the pericardium. Malignancy, independent of other systemic diseases, has also been associated with acute pericardial disease and accounts for about 6% of cases without another explanation . Systemic diseases, such as systemic lupus erythematosus and rheumatoid arthritis , have been found to cause pericardial inflammation with approximately 50% of patients with SLE experiencing pericarditis.
Hypothyroidism severe enough to result in myxedema can result in a large, slowly accumulating pericardial effusion but rarely causes acute pericarditis . Aspirin or NSAIDs are mainstays of therapy for acute pericarditis . If laboratory data support the clinical diagnosis, symptomatic treatment with NSAIDs should be initiated. Because of its excellent safety, the preferred NSAIDs is ibuprofen in a dose of 600 to 800 mg orally, three times daily with discontinuation if pain is no longer present after two weeks . Many patients have very gratifying responses to the first or second dose of NSAIDs, and most respond fully with no need for additional treatment. Reliable patients with no more than small effusions, who respond well to NSAIDs, need not be admitted to hospital .
Patients who do not respond well initially, who have larger effusions, or who have a suspected cause other than idiopathic pericarditis should be hospitalised for additional observation, diagnostic testing and treatment. Patients who respond slowly or inadequately to NSAIDs may require supplementary narcotic analgesics to allow time for a full response or a course of colchicine . Colchicine is recommended at low, weight-adjusted doses to improve the response to medical therapy and to prevent recurrences .
Acute pericarditis is a relatively common cause of acute chest pain which can be readily evaluated by thorough history-taking supplemented by ECG and echocardiography. While the study of pericardial diseases does not receive the same degree of attention as acute coronary syndromes, there is an emerging evidence base for the treatment of idiopathic acute pericarditis. The use of colchicine significantly reduces the chances of recurrence but despite this, a troublesome subset of patients remain who experience multiple recurrences. For this subgroup, there is some promising initial data for the interleukin-1β antagonist anakinra.
Nevertheless, our understanding of the immunopathology and pathogenesis of this pericardial syndrome remains incomplete and more work is required to address this if further therapeutic advances are to be made. Acute pericarditis causes chest pain, which may be very difficult to discern from pain caused by acute myocardial infarction. The chest pain in acute pericarditis may be severe and the patient may also experience cold sweats, tachycardia and anxiety; all of which are common in acute myocardial infarction. Clinical examination may reveal pericardial friction rub and the echocardiogram may show increased fluid in the pericardial cavity . Hemodynamic compromise may occur if accumulation of fluid in the pericardial sac compromises the relaxation and/or contraction of the ventricles and atria.
This situation is referred to as cardiac tamponade, which has been discussed earlier. Symptoms typically include sudden onset of sharp chest pain, which may also be felt in the shoulders, neck, or back. The pain is typically less severe when sitting up and more severe when lying down or breathing deeply. Other symptoms of pericarditis can include fever, weakness, palpitations, and shortness of breath. The onset of symptoms can occasionally be gradual rather than sudden.
The preliminary data published suggest that all myopericarditis cases are mild and clinically resolve within a few days to a few weeks. The bottom line is that the risk of cardiac complications due to SARS-CoV-2 infection far exceeds the minimal and rare risks of vaccination-related transient myocardial or pericardial inflammation. The CDC accepts all reports through VAERS from everyone regardless of the plausibility of the vaccine causing the event or the clinical seriousness of the event. The VRBPAC will follow up on all reported cases to the VAERS and VSD and adjudicate the cases as per the CDC case definition of myocarditis and pericarditis.
Acute myopericarditis associated with COVID-19 vaccine can be associated with arrhythmia. Anticipatory care includes judicious triaging of the disposition of patients seen in ambulatory or emergency setting for work-up or management of probable myopericarditis patients. Whenever possible, serology and PCR for common viral causes of myocarditis such as parvovirus B19, herpesvirus type-6, adenovirus, enterovirus, Epstein–Barr and cytomegalovirus should be obtained to rule out common causes of viral myocarditis. The treatment considerations for COVID-19 vaccine-associated myopericarditis include anti-inflammatory medications and guideline-directed medical therapy if left ventricular function is reduced . No data for any specific treatment for vaccine-associated myocarditis are available. Steroids are used for their potent anti-inflammatory action in cases where the patient has continued symptoms and troponin leak even after NSAIDs.
However, steroids and IVIG are also immunomodulatory and immunosuppressive agents and could reduce the specific immune response against SARS-CoV-2 triggered by the vaccine. Thus, the duration of steroids administration should be limited to the resolution of the symptoms or ventricular arrhythmias or the recovery of the LV function. Among 29 cases with a known outcome, all were discharged to their homes within 1 week, and all made full clinical recoveries. However, the long-term impact of myocardial inflammation following COVID-19 mRNA vaccine as detected on CMR, remains unknown and needs to be systematically evaluated. Further studies are required to elucidate the pathophysiology that underlies this complication to seek mitigation strategies and to delineate optimal therapy. Management is based on discontinuation of the causative agent and symptomatic treatment .
The use of heparin and anticoagulant therapies is a possible risk factor for the development of a worsening or haemorrhagic pericardial effusion that may result in cardiac tamponade. In a study of 274 patients with acute pericarditis or myopericarditis, the use of heparin or other anticoagulants was not associated with an increased risk of cardiac tamponade . On the other hand, in the setting of co-existing pericardial effusion, full anticoagulation may be a risk factor for tamponade and complications .
Rest is usually recommended in acute pericarditis and acute myocarditis. Given that myocarditis often leads to hospitalization, this task seems easy to carry out in hospital practice; however, it could be a real challenge at home in daily life. Heart rate-lowering treatments (mainly beta-blockers) are usually recommended in case of acute myocarditis, especially in case of heart failure or arrhythmias, but level of proof remains weak.
Calcium channel inhibitors and digoxin are sometimes proposed, albeit in limited situations. It is possible that rest or even heart rate-lowering treatments could help to manage these patients by preventing heart failure as well as by limiting "mechanical inflammation" and controlling arrhythmias, especially life-threatening ones. Several questions remain unsolved, such as the duration of such treatments, especially in light of new heart rate-lowering treatments, such as ivabradine. In this review, we discuss rest and heart-rate lowering medications for the treatment of pericarditis and myocarditis.
We also highlight some work in experimental models that indicates the beneficial effects of such treatments for these conditions. Finally, we suggest certain experimental avenues, through the use of animal models and clinical studies, which could lead to improved management of these patients. The most common symptoms of myocarditis in children include fatigue, shortness of breath, abdominal pain and fever.